FORENAP : Votre partenaire clé
"FORENAP est votre levier stratégique"
About Us
FORENAP FRP développe une activité de recherche dans le domaine des neurosciences. A ce titre sont conduites des études en neuropsychopharmacologie et sur les bio-marqueurs dans le but d’étudier les effets de molécules sur le système nerveux central.

Mission
Chez FORENAP, notre mission est de fusionner l’expertise humaine avec les technologies d’intelligence artificielle les plus avancées afin de révolutionner la collecte, l’analyse et l’interprétation des données neurologiques.
Intelligence artificielle et EEG : détecter l’effet d’un traitement en quelques minutes
L’association de l’électroencéphalographie (EEG) et de l’intelligence artificielle ouvre une nouvelle voie pour l’évaluation ultra-rapide de l’effet des traitements psychotropes. Alors que les approches classiques nécessitent plusieurs semaines pour juger de l’efficacité d’un médicament, l’analyse en temps réel des signaux EEG, enrichie par des algorithmes d’apprentissage automatique, permet aujourd’hui de détecter des modifications de l’activité cérébrale dès les premières heures voire minutes suivant l’administration.
Chez FORENAP, nous exploitons des réseaux de neurones profonds pour analyser les signatures électrophysiologiques de patients et de volontaires sains. Ces modèles sont entraînés pour repérer des patterns subtils dans les oscillations cérébrales, indicateurs précoces de la réponse thérapeutique ou de l’apparition d’effets secondaires.
Cette technologie permet une stratification rapide des patients, une optimisation des essais cliniques et, à terme, une personnalisation des traitements en psychiatrie et neurologie. Grâce à cette approche, nous contribuons à transformer la manière dont les médicaments agissant sur le système nerveux central sont évalués et développés.

Cartographier les effets des molécules sur l’activité cérébrale
Comprendre comment une molécule agit sur le cerveau nécessite plus qu’une simple évaluation clinique : cela implique de visualiser, en temps réel, les modifications qu’elle induit au cœur des réseaux neuronaux. Chez FORENAP, nous mobilisons des outils de neuroimagerie de pointe IRM fonctionnelle et EEG haute résolution pour cartographier les dynamiques cérébrales avec une précision spatio-temporelle exceptionnelle.
L’IRMf permet de suivre les variations du flux sanguin cérébral , reflet de l’activité neuronale, afin d’observer quelles régions s’activent ou se désactivent sous l’effet d’un traitement. En parallèle, l’EEG capture les rythmes électriques du cerveau à la milliseconde près, révélant comment les molécules influencent les circuits neuronaux à travers différents états cognitifs.
Cette double approche permet de générer des profils pharmacodynamiques cérébraux complets, et d’identifier des biomarqueurs d’activité fonctionnelle, essentiels pour évaluer l’efficacité ou le mécanisme d’action d’un nouveau traitement. Nos protocoles intègrent aussi des analyses en intelligence artificielle pour extraire des signatures spécifiques invisibles à l’œil humain.
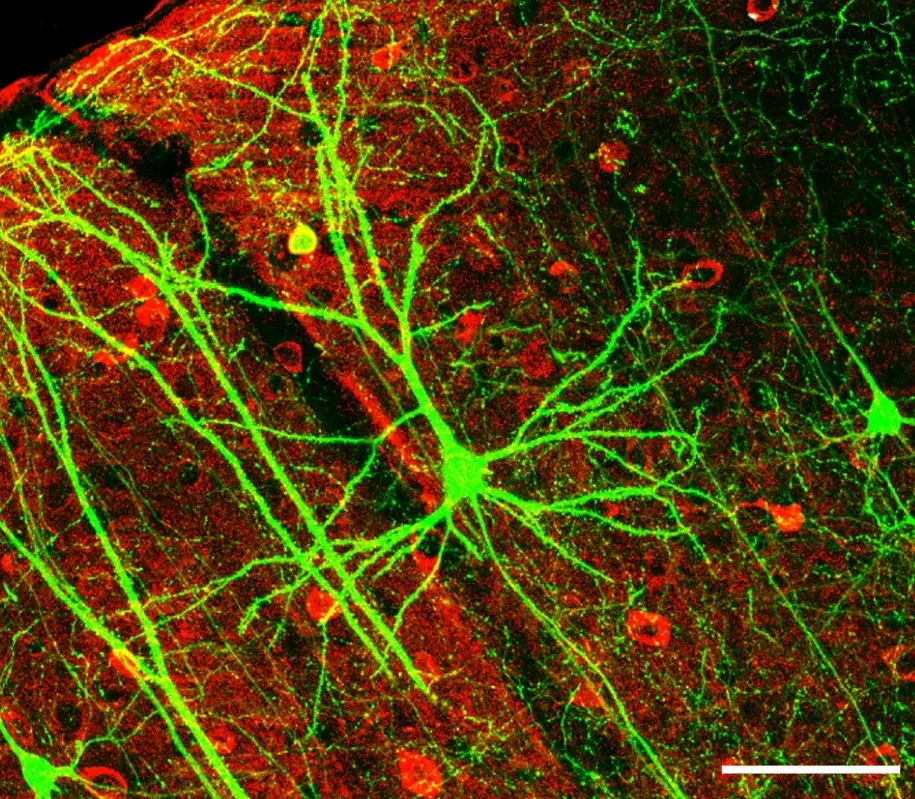
Biomarqueurs et intelligence des soins : vers des traitements ciblés du cerveau
La complexité des troubles neurologiques et psychiatriques exige une nouvelle manière de penser la prise en charge thérapeutique : plus personnalisée, plus prédictive, et fondée sur des données biologiques objectives. À la croisée des neurosciences, de la biologie et de l’intelligence artificielle, FORENAP développe des approches basées sur l’identification de biomarqueurs fiables, capables de guider les décisions cliniques et de concevoir des traitements ciblés, adaptés à chaque profil cérébral.
Ces biomarqueurs qu’ils soient moléculaires, électrophysiologiques (EEG), inflammatoires, génétiques ou issus de l’imagerie fonctionnelle permettent de mieux comprendre les mécanismes spécifiques à chaque patient, d’anticiper la réponse à un traitement, ou encore de détecter précocement les signaux de rechute. En intégrant ces données à des algorithmes d’analyse avancés, nous construisons des modèles prédictifs de réponse thérapeutique, au service d’une médecine de précision du cerveau.
Cette intelligence des soins transforme les protocoles traditionnels en approches dynamiques, adaptatives et fondées sur des preuves.
Précision thérapeutique en neurosciences : le rôle clé des biomarqueurs
Les biomarqueurs transforment profondément la prise en charge en neurosciences. En fournissant des indicateurs biologiques objectifs qu’ils soient moléculaires, neurophysiologiques (EEG), inflammatoires ou issus de l’imagerie cérébrale ils permettent de stratifier les patients, de prédire la réponse aux traitements et de personnaliser les interventions thérapeutiques.
Intégrés à des modèles d’analyse avancés, ces marqueurs rendent possible une médecine de précision, fondée non plus sur les seuls symptômes, mais sur les mécanismes biologiques sous-jacents. Ce changement de paradigme permet d’optimiser les protocoles, de réduire les essais-erreurs et d’améliorer l’efficacité des soins dans les troubles neurologiques et psychiatriques.
Neurosciences de précision soigner en fonction du profil biologique :
Les approches classiques en neurologie et en psychiatrie reposent souvent sur des traitements standardisés, appliqués à des populations hétérogènes. Or, les recherches récentes montrent que chaque individu présente un profil biologique unique, influencé par des facteurs génétiques, moléculaires, électrophysiologiques et environnementaux. C’est cette singularité que les neurosciences de précision visent à exploiter pour adapter les traitements à la biologie du patient, et non l’inverse.
En s’appuyant sur des biomarqueurs objectifs tels que les signatures EEG, les niveaux de cytokines, les profils génétiques ou l’activité cérébrale observée par IRM fonctionnelle il devient possible de stratifier les patients en sous-groupes biologiquement cohérents. Ces données permettent de prédire la réponse à un traitement, de détecter des formes spécifiques de résistance, et de choisir l’intervention thérapeutique la plus appropriée, qu’elle soit pharmacologique, comportementale ou technologique (stimulation cérébrale, neurofeedback).
Cette approche transforme la logique du soin en neurosciences : elle réduit les essais-erreurs, améliore la tolérance des traitements et accélère les réponses cliniques. En combinant données biologiques, intelligence artificielle et expertise clinique, les neurosciences de précision ouvrent la voie à une médecine cérébrale personnalisée, prédictive et mécanistique, où chaque décision est guidée par les caractéristiques neurobiologiques propres à l’individu.